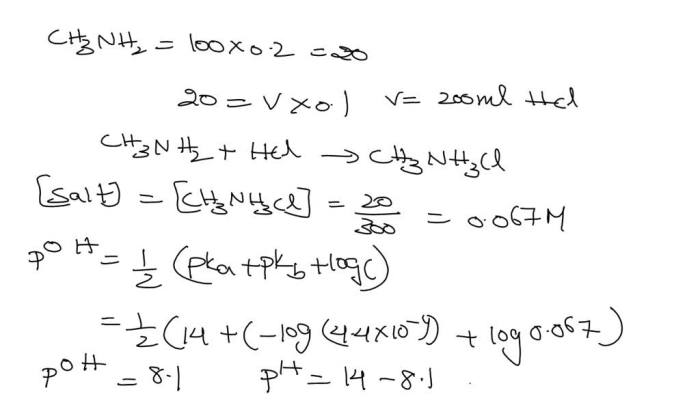Unveiling the Principles of General Chemistry 2nd Edition Solutions, this comprehensive guide unlocks the fundamental concepts and theories that govern the world of chemistry. Embark on a journey through the intricacies of chemical bonding, thermodynamics, equilibrium, acids, bases, redox reactions, and organic chemistry, gaining a deeper understanding of the natural world.
With a focus on clarity and engagement, this guide provides a comprehensive overview of the major concepts covered in the textbook, illuminating their significance in understanding chemical phenomena and their applications in various fields.
Key Concepts and Theories
The second edition of the textbook “Principles of General Chemistry” presents the fundamental principles of general chemistry in a clear and concise manner. It provides a comprehensive overview of the major concepts covered in the book, including atomic structure, chemical bonding, thermodynamics, kinetics, equilibrium, solutions, acids, bases, and salts, redox reactions, electrochemistry, and organic chemistry.
These concepts are essential for understanding the behavior of matter and the chemical reactions that occur in the natural world. The book emphasizes the importance of these concepts in various fields such as medicine, engineering, and environmental science.
Chemical Bonding and Structure
Types of Chemical Bonds
The book discusses the various types of chemical bonds, including ionic bonds, covalent bonds, and metallic bonds. It explains the properties of each type of bond and how they determine the structure and properties of molecules.
Relationship between Molecular Structure and Bonding
The book emphasizes the relationship between molecular structure and bonding. It explains how the arrangement of atoms in a molecule affects its properties, such as its polarity, solubility, and reactivity.
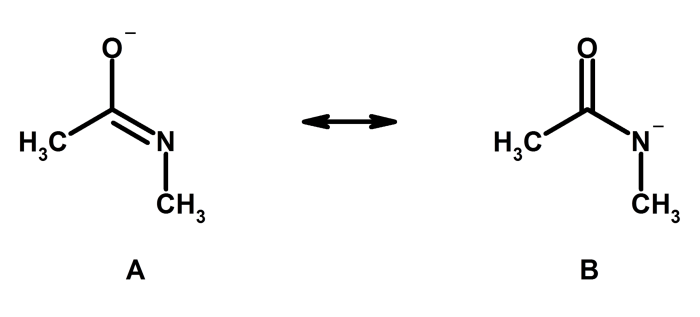
Resonance
The concept of resonance is also discussed in the book. Resonance is a phenomenon that occurs when a molecule can be represented by two or more valid Lewis structures. The book explains how resonance affects the properties of molecules.
Thermodynamics and Kinetics

Definition of Thermodynamics
The book defines thermodynamics as the study of energy and its transformations. It explains the laws of thermodynamics and how they can be used to predict the spontaneity of chemical reactions.
Enthalpy, Entropy, and Free Energy
The book discusses the concepts of enthalpy, entropy, and free energy. It explains how these concepts are related to the spontaneity of chemical reactions.
Chemical Kinetics
The book describes the factors that influence reaction rates and discusses the theories of chemical kinetics. It explains how reaction rates can be measured and how they can be used to predict the outcome of chemical reactions.
Equilibrium and Solutions
Chemical Equilibrium
The book defines chemical equilibrium as a state in which the concentrations of the reactants and products of a chemical reaction do not change over time. It explains the factors that affect chemical equilibrium.
Equilibrium Constants, Principles of general chemistry 2nd edition solutions
The book discusses the different types of equilibrium constants and their applications. It explains how equilibrium constants can be used to predict the extent of a chemical reaction.
Solubility and Colligative Properties
The book explains the concepts of solubility and colligative properties. It discusses how solubility can be affected by various factors and how colligative properties can be used to determine the molecular weight of a solute.
Types of Solutions
The book describes the various types of solutions, including homogeneous solutions, heterogeneous solutions, and colloidal solutions. It explains the properties of each type of solution.
General Inquiries: Principles Of General Chemistry 2nd Edition Solutions
What are the key concepts covered in the Principles of General Chemistry 2nd Edition Solutions?
The key concepts covered include chemical bonding, thermodynamics, equilibrium, acids, bases, redox reactions, and organic chemistry.
How does the guide explain the significance of these concepts?
The guide provides clear and engaging explanations of each concept, highlighting their importance in understanding chemical phenomena and their applications in various fields.
What are the benefits of using the Principles of General Chemistry 2nd Edition Solutions?
The guide offers a comprehensive overview of the textbook’s content, enhancing understanding and providing a valuable resource for students, educators, and professionals.