Select the true statements about the resonance structures – Select the true statements about resonance structures and delve into the fascinating realm of chemistry, where molecules dance in a delicate balance, revealing their secrets through the interplay of resonance. This concept, fundamental to understanding molecular behavior, unveils the intricacies of chemical bonding, molecular stability, and a plethora of applications that shape our world.
Resonance structures, like snapshots of a dynamic molecular landscape, provide a glimpse into the true nature of molecules. They capture the essence of electron delocalization, a phenomenon where electrons roam freely across multiple atoms, blurring the boundaries of traditional chemical bonds.
This dance of electrons not only influences molecular geometry but also dictates reactivity and a myriad of other properties that govern the behavior of matter.
Resonance Structures Basics
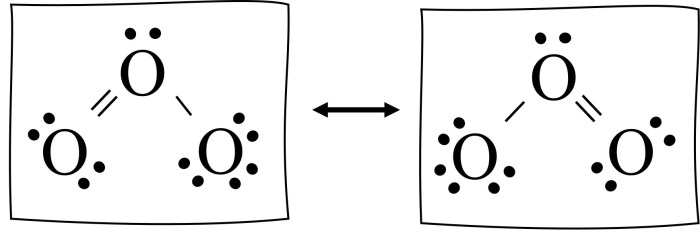
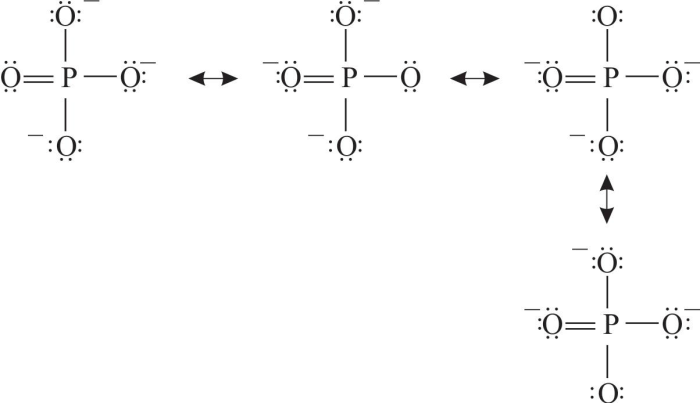
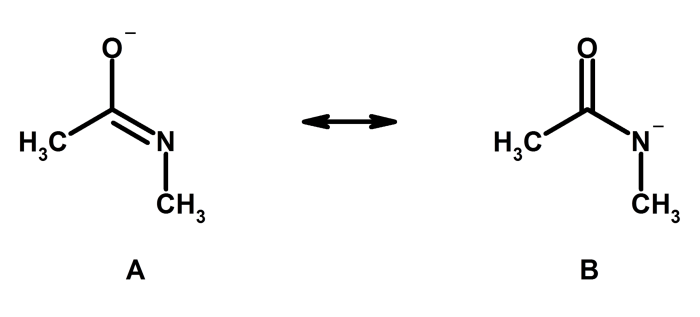
Resonance structures are hypothetical representations of a molecule that differ only in the arrangement of electrons. They are used to describe the electronic structure of molecules that cannot be adequately represented by a single Lewis structure. Resonance structures are significant because they provide a more accurate picture of the electronic structure of a molecule and can be used to explain a variety of molecular properties.
For example, the benzene molecule has six carbon atoms arranged in a ring, with one hydrogen atom attached to each carbon atom. The Lewis structure of benzene shows each carbon atom bonded to its neighboring carbon atoms by a single bond and to its hydrogen atom by a single bond.
However, this Lewis structure does not accurately represent the electronic structure of benzene. In reality, the electrons in the benzene ring are delocalized, meaning that they are not confined to a single bond between two atoms. This delocalization of electrons is represented by drawing resonance structures for benzene.
The two resonance structures for benzene show the electrons in the ring alternating between single and double bonds.
The resonance structures for benzene can be used to explain a variety of molecular properties. For example, the resonance structures show that the benzene ring is aromatic, which means that it is unusually stable. The stability of benzene is due to the delocalization of electrons, which lowers the energy of the molecule.
Identifying Resonance Structures
There are a number of criteria that can be used to identify resonance structures. First, the resonance structures must have the same number of electrons. Second, the resonance structures must have the same number of atoms of each type. Third, the resonance structures must have the same overall charge.
Finally, the resonance structures must be connected by a single bond.
There are a number of methods that can be used to generate resonance structures for a given molecule. One method is to use the Lewis structure of the molecule. The Lewis structure can be used to identify the atoms that are bonded to each other and the number of electrons that are involved in each bond.
This information can then be used to generate resonance structures for the molecule.
Another method that can be used to generate resonance structures is to use the molecular orbital theory. The molecular orbital theory can be used to calculate the energy of the electrons in a molecule. This information can then be used to generate resonance structures for the molecule that have the lowest energy.
Resonance and Molecular Properties
Resonance can affect a variety of molecular properties. For example, resonance can affect the bond lengths, bond angles, and dipole moments of a molecule. Resonance can also affect the reactivity of a molecule.
For example, the resonance structures for benzene show that the bond lengths between the carbon atoms in the ring are all equal. This is because the electrons in the ring are delocalized, which means that they are not confined to a single bond between two atoms.
The delocalization of electrons also results in the benzene ring having a planar structure. This is because the electrons in the ring can move freely around the ring, which prevents the ring from bending.
Resonance can also affect the reactivity of a molecule. For example, the resonance structures for benzene show that the benzene ring is aromatic. This means that the benzene ring is unusually stable and does not react easily with other molecules.
Applications of Resonance, Select the true statements about the resonance structures
Resonance is a powerful tool that can be used to understand a variety of molecular properties and reactions. Resonance is used in a variety of areas of chemistry, including organic chemistry, inorganic chemistry, and physical chemistry.
In organic chemistry, resonance is used to explain the stability of aromatic compounds. In inorganic chemistry, resonance is used to explain the bonding in metal complexes. In physical chemistry, resonance is used to explain the electronic structure of molecules and to calculate molecular properties.
Resonance is also important in the design and development of new materials. For example, resonance is used to design new materials that are stronger, lighter, and more efficient. Resonance is also used to design new materials that have specific electronic properties, such as semiconductors and superconductors.
Helpful Answers: Select The True Statements About The Resonance Structures
What is the significance of resonance structures?
Resonance structures provide a deeper understanding of molecular bonding and stability by depicting the delocalization of electrons, which influences molecular geometry, reactivity, and other properties.
How can we identify resonance structures?
Resonance structures can be identified by considering the following criteria: the presence of multiple Kekule structures, equivalent atoms or groups, and the absence of formal charges on adjacent atoms.
What is the relationship between resonance and molecular stability?
Resonance contributes to molecular stability by delocalizing electrons, which lowers the overall energy of the molecule. The more resonance structures a molecule has, the more stable it is.